Research & Development at SAN Vet
Our passion for innovation is the driver for our R&D activities at SAN Vet and SAN Group as a whole. Besides our highest standards in customer service and quality, our in-house R&D is another strong cornerstone of our success.
Research
Research on animal health represents one of the core competencies of SAN Group. This results in competitive market solutions through innovation. Research is also a tool to reduce risks related to high investments required by the maintenance and registration of new products. As in all processes, research features highly qualified specialists and experts. Through partnerships with respected laboratories, universities, and worldwide research institutions, SAN Vet is constantly engaging with new scientific knowledge in animal health.
Development
Development at SAN Vet scouts trends and predicts demands, develops innovative solutions, supports the technical teams and customers in the animal health market. To stay on top of the latest trends, the members of our development teams participate in national and international conferences and are in close contact with our customers. Additionally, we strongly network with universities, authorities, and industrial partners.
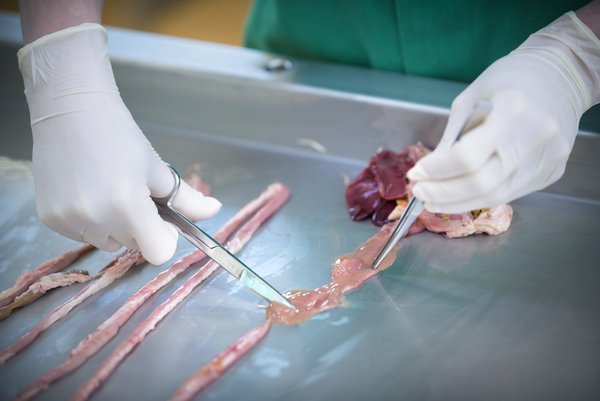
Veterinary Diagnostics
ANICON
ANICON strives to be at the apex of scientific advances, continuously monitoring, testing, and implementing new and significant developments in diagnostic methodologies. These methodologies comprise pathology, histopathology, bacteriology, microbiology, virology, serology, and molecular diagnostics including real-time PCR and sequencing. We ensure that all diagnostic needs of our customers, from regular pathogen isolation or serological monitoring up to highly specialized services for pathogens of particular interest, are met. Development at ANICON branches from a multi-disciplinary team of veterinarians, life science specialists, and biochemical as well as bioinformatic engineers.
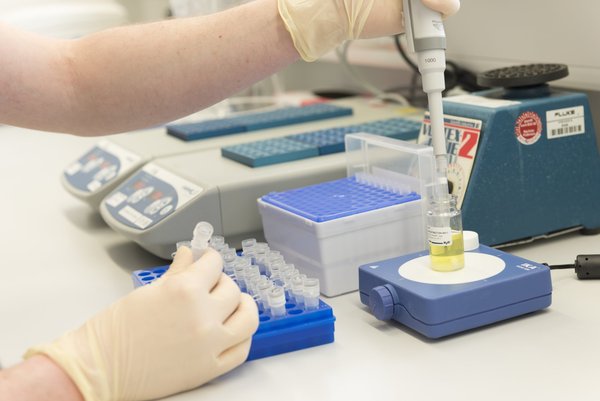
In-Vitro Diagnostics
KYLT
At KYLT we continuously design and complement our products to provide the most convenient, reliable, and state-of-the-art diagnostic experience. Today KYLT offers one of the most comprehensive portfolios of real-time PCR kits for detection, typing, and characterization of pathogens in poultry, swine, ruminants, and aquaculture. The PCR kit portfolio is supplemented by products for sampling, sample conservation, sample preparation, and automatization of sample preparation and PCR setup. We also developed our own KYLT software for the most convenient application of our kits. Development at KYLT stems from the expertise of a multi-disciplinary team of scientific experts, veterinarians, sales, and technical support specialists as well as bioinformatic engineers. This mix of professionals enables us to offer exceptionally fast and reliable support to all of our customers, including hands-on-training.
Vaccines
ANIVAC
We are one of the few manufacturers of autogenous vaccines in Europe. With our production facility inaugurated in November 2022 in Northern Germany, we already provide a production quality standard in line with the Good Manufacturing Practice (GMP) requirements expected for autogenous vaccines as per Regulation (EU) 2019/6. This makes our products safe as well as efficient, which is why our customers can fully rely on the high quality of our autogenous vaccines. In addition to production, we always keep a close eye on the market to improve and adapt our products in terms of efficacy and formulation. Moreover, we constantly stive to meet our customers’ needs for new products to fill potential gaps in animal illness prevention. The team consists of highly specialized and experienced veterinarians, life science specialists, biochemical engineers, experts in quality management and control as well as highly qualified and experienced technical staff. We are part of the EMAV e.V. (European Manufacturers of Autogenous Vaccines and Sera association), which allows us to also stay in close contact with political regulators and authorities, thus remaining up to date in all matters related to autogenous vaccine production.
Biosecurity & Medicals
SANPHAR
The SANPHAR development is based on two main pillars: our state-of-the-art research laboratories and trial facilities in Austria and Brazil in addition to numerous development partners worldwide. The practice of “open innovation” between institutes, universities, entrepreneurs, start-ups in biotechnology, and our own teams has proven to accelerate innovative projects. A multi-disciplinary team of veterinarians and animal scientists execute in-vitro and field trials, publish scientific papers, and support product management in product positioning, the creation of technical and marketing material, as well as launching new products and services. To also keep our colleagues in the field updated on technical novelties, our team conducts trainings for our sales and technical experts as well as customers.